Background
Cervical remodeling, the process by which the cervix undergoes structural and biochemical changes in preparation for childbirth, offers valuable insights into identifying the risk of preterm birth. During pregnancy, the cervix transitions from a firm and closed state to a softer and more dilated condition, allowing for the passage of the fetus during delivery. Abnormalities or deviations from this typical remodeling process can indicate an increased risk of preterm birth. Various methods, such as transvaginal ultrasound, digital examinations, and biomarker analysis, can assess cervical length, consistency, and other parameters to gauge the extent of cervical remodeling. For instance, a shortened cervical length or changes in the cervical mucus composition may suggest a heightened risk of preterm labor. Additionally, advancements in imaging technologies have enabled more accurate and early detection of cervical changes associated with preterm birth risk. By monitoring cervical remodeling throughout pregnancy, healthcare providers can identify high-risk individuals and implement targeted interventions to mitigate the likelihood of preterm birth, thus improving maternal and neonatal outcomes.
Mouse Models
In our laboratory, we leverage a multidimensional imaging approach to explore cervical remodeling dynamics during healthy pregnancies in mouse models. Our primary techniques include Mueller matrix imaging, second harmonic generation (SHG), and two-photon fluorescence microscopy (TPF). Mueller matrix imaging allows us to probe the structural and optical properties of cervical tissues with high sensitivity, providing valuable information about collagen fiber organization and orientation. Second harmonic generation enables us to specifically visualize collagen fibers due to their nonlinear optical properties, offering insights into their structural arrangement and changes over gestation. Additionally, two-photon fluorescence microscopy provides depth-resolved imaging capabilities, allowing us to observe cellular and extracellular components within the cervix without needing exogenous labels. By integrating these imaging modalities, we can comprehensively assess mechanical properties, collagen remodeling, and tissue microstructure throughout pregnancy, shedding light on the biomechanical adaptations of the cervix. This holistic approach enhances our understanding of cervical remodeling processes and their implications for preterm birth risk assessment.
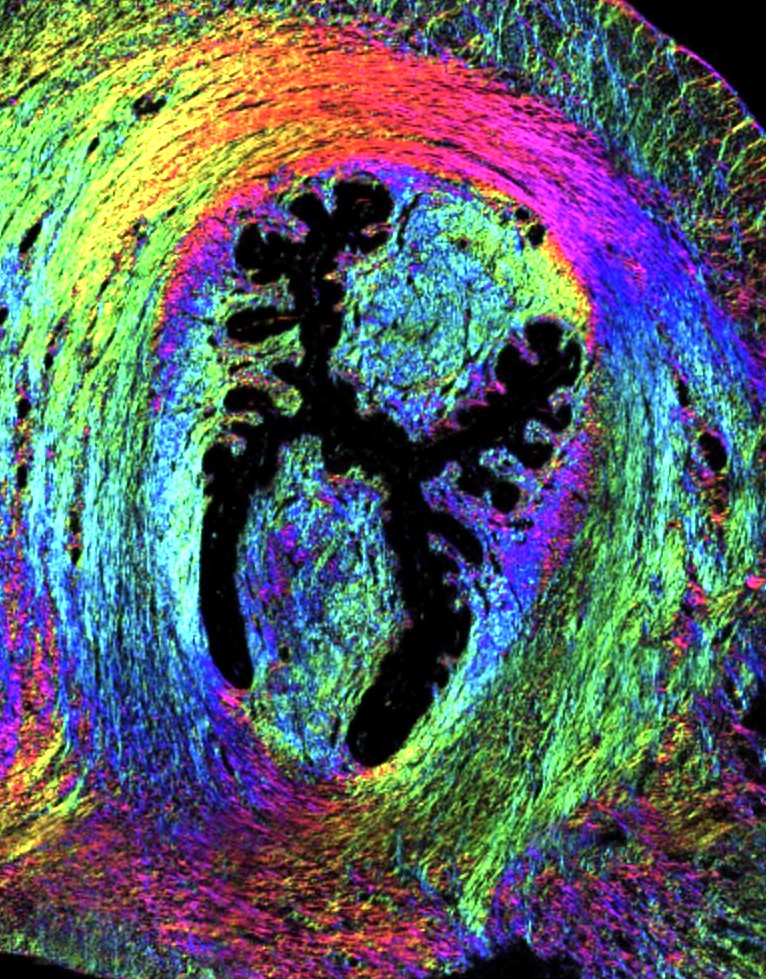
This image displays a high-resolution view of mouse cervical tissue, showcasing how advanced imaging technologies are applied in animal models to understand human conditions better. It captures the detailed architecture of collagen fibers and cellular structures within the cervix. Techniques such as Mueller matrix imaging, second harmonic generation, and two-photon fluorescence microscopy are employed to provide precise measurements of tissue properties and structural changes during pregnancy. This image exemplifies the integration of multiple imaging modalities to enhance the understanding of tissue mechanics and remodeling. These studies are critical for developing insights into the mechanisms of cervical changes, highlighting the crucial role of imaging animal models to assess the risk of preterm birth and advancing clinical research.
Clinical Applications
In parallel with our investigations using mouse models to understand cervical remodeling during healthy pregnancies, our laboratory has pioneered the development of the Portable Preterm Imaging System (PPRIM). This innovative technology represents a significant leap forward in cervical health assessment. Encased within a flexible insertable sheath, the PPRIM houses a specialized imaging setup designed to non-invasively evaluate cervical tissue. One of its primary functions is to analyze the structural integrity of collagen fibers within the cervix, a critical factor in maintaining pregnancy to term. By leveraging advanced imaging techniques such as polarization imaging, the PPRIM can precisely quantify the organization and strength of cervical collagen networks. This capability enables early detection of deviations from normal collagen architecture, which may signal an increased risk of preterm birth. Consequently, healthcare providers can implement timely interventions to mitigate this risk, potentially extending the duration of pregnancy and improving outcomes for both mothers and infants. As we continue to refine and validate the PPRIM through ongoing research, its integration into clinical practice holds promise for revolutionizing prenatal care and reducing the burden of preterm birth worldwide.

Relative Publications
Interested? How can you get involved!
Interested or just looking for more information? Please see our listed publications for more on specific topics. MPL offers opportunities with FIU and our summer program in this topic and related areas.