 |
|||||
| Home | Research | For Teachers | HISTORY Level 1 Level 2 Level 3 |
PRINCIPLES Level 1 Level 2 Level 3 |
CAREER Level 1 Level 2 Level 3 |
| Gallery | Hot Links | What's New! | |||
| Web Administration and Tools | |||||
 |
|||||
| Home | Research | For Teachers | HISTORY Level 1 Level 2 Level 3 |
PRINCIPLES Level 1 Level 2 Level 3 |
CAREER Level 1 Level 2 Level 3 |
| Gallery | Hot Links | What's New! | |||
| Web Administration and Tools | |||||
![]()
Energy is the force behind the movement of all things. Animals (including humans) use food as their energy source for life and movement. Mechanical or inanimate objects use fuel energy to perform work. What is energy? Let's review what you probably have already learned in school. The word ENERGY as defined in physics, is "the capacity for doing work and overcoming resistance." Unless it is doing work, energy is known as potential energy (stored energy). The fuel (usually gasoline) used to power reciprocating engines is potential energy up to the moment it is mixed with air (oxygen) and burned.
When potential energy is released from its source and causes movement of an object, it becomes kinetic energy (active energy). Thus. the movement of the parts of a reciprocating engine is an example of the potential energy of the fuel having been changed to kinetic energy.
Potential and kinetic are broad classifications of energy. Energy is also given several other titles depending on the form it is in at a given moment. That is, energy can be changed from one form to another, so various titles are used to describe the forms. As examples: Heat energy can be changed into mechanical energy; mechanical energy can be changed into electrical energy; and electrical energy can be changed into heat, mechanical, or light energy.
Boyle's law states that the volume of a gas varies inversely with the pressure on it (see figure 6-1 ). This means that any confined gas will double its pressure if its volume is decreased by one-half. If we have a cylinder in which ordinary air is present at 14.7 pounds per square inch (psi) and we rammed an airtight piston into the cylinder one-half the length of the cylinder, the pressure of the gas, or air, would double to 29.4 psi. Then, if we were to ram the piston an additional one-half of the remaining distance in the cylinder, the pressure would increase to 58.8 psi.
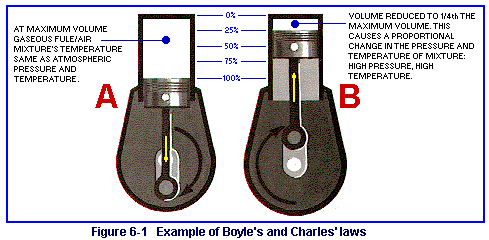
What happens when the reverse is tried? Let's suppose that we begin with the piston in one-half the length of the cylinder. (The pressure within the cylinder is 14.7 psi.) If the piston were extracted quickly to the full length of the cylinder, what do you think would happen to the pressure within the cylinder? It would be reduced by one-half, to become 7.35 psi. We can say this another way. When the volume of a confined gas is doubled, its pressure is reduced by one-half.
As a general summary of Boyle's law, you should remember that a decrease in volume causes an increase in pressure. An increase in volume causes a decrease in pressure.
When the piston in a cylinder moves inward and outward, increasing and decreasing the pressure of a confined gas, what is happening to the temperature of the gas? Charles' law states, the pressure and temperature of a confined gas are directly proportional. Thus, when the piston in a cylinder moves inward and compresses the gas, the temperature of the gas increases. How much the temperature increases depends on how far the piston t ravels.
While an aircraft engine is operating, these two laws are being applied. It is through the understanding of these and related laws of physics that engineers have been able to improve the efficiency of engines.
Send all comments to ![]() aeromaster@eng.fiu.edu
aeromaster@eng.fiu.edu
© 1995-98 ALLSTAR Network. All rights reserved worldwide.
| Funded in part by | From Civil Air Patrol Educational Materials |
Updated: February 23, 1999