 |
|||||
| Home | Research | For Teachers | HISTORY Level 1 Level 2 Level 3 |
PRINCIPLES Level 1 Level 2 Level 3 |
CAREER Level 1 Level 2 Level 3 |
| Gallery | Hot Links | What's New! | |||
| Web Administration and Tools | |||||
 |
|||||
| Home | Research | For Teachers | HISTORY Level 1 Level 2 Level 3 |
PRINCIPLES Level 1 Level 2 Level 3 |
CAREER Level 1 Level 2 Level 3 |
| Gallery | Hot Links | What's New! | |||
| Web Administration and Tools | |||||
![]()
Density is illustrated in Figure 2-2a and is the amount of material contained in a unit of volume. Density is constant in solids because more material cannot be forced into a given volume of a solid. Solids will remain almost unchanged in density as long as the temperature doesn't become high enough to cause the solids to melt or burn. With air, however, the story is quite different.

Density: Amount of material PER UNIT VOLUME.
Figure 2-2a shows two small one-inch cubes on the right side. One of them weighs only one pound, and the other, with tightly packed molecules, weighs ten pounds Although the volume is the same, the weight is very different because the molecules are packed closer together. The density of the air and the changes that temperature produce upon the density of air play an important role in an aircraft's flight.
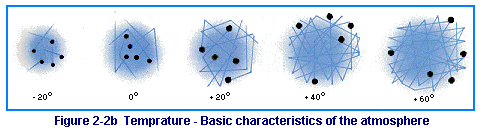
Temperature: ENERGY of motion.
Since air is a gas, it is free to expand or contract as its temperature changes. Notice in Figure 2-2b how the five molecules of air increase their range of motion with each 20-degree increase in temperature. Since the molecules are not confined in a container, the air expands as the temperature increases. This also means that the range of particle motion decreases with decreases in temperature. Comparing the temperature and density of a unit of air, you can see that as temperature increases the density will decrease, and as the temperature decreases the density increases. As you will understand later, this constantly changing air density is very significant to flight.
Send all comments to ![]() aeromaster@eng.fiu.edu
aeromaster@eng.fiu.edu
© 1995-98 ALLSTAR Network. All rights reserved worldwide.
| Funded in part by | From Civil Air Patrol Educational Materials |
Updated: 23 February, 1999